Politicians playing doctor
by Sandy Szwarc
Gov. Abbott came to sell a new antibody therapy in Lubbock. It turns out he’s marketing Eli Lilly’s therapy, bemlanivimab (not Regeneron’s, as originally believed).
Why is a politician promoting a drug? Shouldn’t medical care be driven by solid science, not politics?
It is seriously doubtful that the media will report the full story. How many doctors will even actually pull up the research and all of the supplemental documents?
Like Regeneron’s, this therapy is only being tested in people who get a positive pcr test for Covid-19, but who aren’t especially sick or needing hospitalization or oxygen. It’s being released for use based on the phase 2 clinical trial (Blaze-1) that has not been completed. Its completion date is scheduled for March 11, 2021. An interim analysis was released in a press release in October and published in New England Journal of Medicine a few weeks ago.
This treatment is considered experimental and only permitted under an FDA Emergency Use Authorization.
Medical practice and clinical care guidelines are traditionally based on the completion of randomized, controlled clinical PHASE THREE trials, with a body of evidence showing safety and efficacy – not on a phase 2 study, let alone a single one that hasn’t even been completed and only interim data released. Phase 2 trials are done on a relatively small group of people and are fairly short term; their primary goal is to determine the safety of the drug. [The Difference between Phase I, II, & III Trials]
The primary endpoint of this phase 2 study was to see if it reduced viral load; secondary endpoints were symptom related. It’s unknown if it actually shortened the duration of illness from the virus, which it doesn’t appear to have.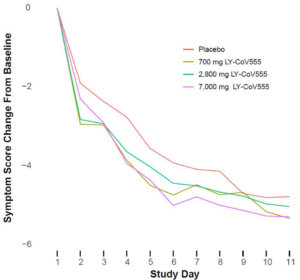
Any objective medical analysis will find several points of concern, but a few that should have jumped out to any doctor:
- Viral load reduction was completely unrelated to the drug dosage (jumping around at different doses), which is a big red flag.Combining the different doses, they showed a difference of 0.24 mg from the placebo group at day 11, for example. The placebo group, however, had a larger, natural reduction in viral load than the treatment group receiving the highest dose. The changes in viral loads throughout those eleven days between those getting the drug at any dose and the placebo group were so similar, any real clinical difference would be questionable.
- It touted an 84.5% lower relative risk of hospitalizations. That’s a red flags.First, anytime you see relative risk instead of absolute risks reported in a press release or study, you are seeing marketing. The actual difference was a total of 4 patients.
- Side effects (“adverse events”) reported in the patients getting the drug as compared to the placebo group were significantly higher − two to four times higher, in fact! Nausea and vomiting, dizziness, diarrhea, itching, chills, chest pain, high blood pressure, rash, etc. Of course, if you’re part of an experimental trial and experience any adverse effects of concern, they will send you to the ER to be checked out. Although the actual numbers were small, severe adverse events were 5 times higher among those getting the drug as compared to the placebo group. Why didn’t the headlines trumpet “a 500% increase in severe adverse events!”?
The total symptom scores (symptom questionnaires completed by the patients) during the study differed between the drug and placebo groups by tiny percentages.
This is why we wait to see the results of full clinical trials, preferably phase three trials, before drugs are approved for use.
Sandy Szwarc, BSN, RN is a graduate of U.T. Austin and a researcher and writer on health and science issues for more than 30 years.
A few other resources:
- https://endpts.com/a-p-value-of-0-38-nejm-results-raise-new-questions-for-eli-lillys-vaunted-covid-antibody/
- https://endpts.com/eli-lilly-lines-up-a-blockbuster-deal-for-covid-19-antibody-right-after-it-failed-a-niaid-trial/
- https://seekingalpha.com/article/4383527-latest-antibody-data-from-lilly-and-from-regeneron
- https://blogs.sciencemag.org/pipeline/archives/2020/10/27/more-antibody-data




















Thank you so much for sharing this, Mr. Pratt. I am concerned for people and that some patients may sign up based on the press release (from the drug company’s investor site) and the media stories, and they may not realize the drug is still at an early experimental phase.
It’s one thing for a terminal patient, with few treatment options left, to sign up for an early clinical trial, it’s quite another for someone to do so if they have a 99% chance of recovering. Patients deserve to have all information and perspectives from all sides, so that they can weigh the risks and benefits and can make an informed decision about what is best for them.
The interim report that was published failed to describe the hospitalizations so there is no way to know if the study participants went to the hospital because of advancing Covid symptoms or because of adverse effects of the drug (the authors lumped them together). We don’t know the severity of their illness or duration of hospitalization.
We simply have insufficient information based on the available data at this point, I believe. It’s always worrisome when the marketing seems overstated to the evidence.
Yes, and given such scant data and even need for this drug for the cohort it is designed for, I truly wonder why Abbott is schilling for Eli Lilly and on our dime to do it.